Chapter 1 of Class 10 Chemistry Chemical Reactions and Equations is one of the most important and high-scoring chapters in the CBSE syllabus. This chapter explains the basics of chemical reactions, how to write chemical equations, and how to balance them correctly. A strong understanding of these concepts helps students perform better in board exams and builds a solid foundation for higher-level chemistry.
These Class 10 Chemistry Chapter 1 quick revision notes are written in simple, easy-to-understand language so every student can revise quickly without confusion. The notes cover key topics such as types of chemical reactions, balanced chemical equations, oxidation and reduction, corrosion and rancidity, along with important examples frequently asked in exams.
Introduction to Chemical Reactions
Chemical Reaction: A process in which new substances with different chemical properties are formed by the breaking and making of bonds between atoms.
Daily Life Examples
- Milk left at room temperature in summer → turns sour (lactic acid formed).
- Iron tawa/nail in humid air → rusts (Fe₂O₃·xH₂O).
- Grapes get fermented → alcohol + CO₂.
- Food is cooked → new substances (Maillard reaction).
- Food digested in the body → simpler molecules + energy.
- We respire → glucose + O₂ → CO₂ + H₂O + energy.
How do we know a chemical reaction has occurred?
- Change in state (solid → liquid/gas)
- Change in colour
- Evolution of gas (bubbles/fizzing)
- Change in temperature (heat released/absorbed)
Activity 1.1 (Teacher-assisted):
Burn Mg ribbon → dazzling white flame + white powder (MgO).
Observation: Magnesium + Oxygen → Magnesium oxide.
Activity 1.2: Pb(NO₃)₂(aq) + KI(aq) → yellow ppt of PbI₂.
Activity 1.3: Zn granules + dil. H₂SO₄/HCl → bubbles of H₂ gas + test tube becomes hot.
Chemical Equations
Word Equation (long):
Magnesium + Oxygen → Magnesium oxide
Chemical Equation (short form):
Mg + O₂ → MgO (Skeletal/Unbalanced)
Balanced Chemical Equation obeys Law of Conservation of Mass
Total mass of reactants = Total mass of products
The number of atoms of each element is the same on both sides.
Step-by-Step Balancing (Hit-and-Trial Method)
Example: Fe + H₂O → Fe₃O₄ + H₂
Step I: Draw boxes → Fe + H₂O → Fe₃O₄ + H₂
Step II: Count atoms
| Element | LHS | RHS |
| Fe | 1 | 3 |
| H | 2 | 2 |
| O | 1 | 4 |
Step III: Balance oxygen (most complex compound Fe₃O₄) → Fe + 4H₂O → Fe₃O₄ + H₂
Step IV: Balance hydrogen → Fe + 4H₂O → Fe₃O₄ + 4H₂
Step V: Balance iron → 3Fe + 4H₂O → Fe₃O₄ + 4H₂
Final Balanced: 3Fe(s) + 4H₂O(g) → Fe₃O₄(s) + 4H₂(g)
Physical States (must write in 3/5 mark answers):
- (s) Solid
- (l) Liquid
- (g) Gas
- (aq) Aqueous (dissolved in water)
Reaction Conditions (above/below arrow):
- Heat △, Sunlight, Electricity, Catalyst, 340 atm, etc.
Example (1.11): CO(g) + 2H₂(g) →[340 atm] CH₃OH(l)
Example (1.12): 6CO₂(aq) + 12H₂O(l) →[Sunlight + Chlorophyll] C₆H₁₂O₆(aq) + 6O₂(aq) + 6H₂O(l)
Types of Chemical Reactions
Combination Reaction
Two or more substances → single product
General: A + B → AB
Activity 1.4: CaO (quick lime) + H₂O → Ca(OH)₂ (slaked lime) + Heat (exothermic)
Equation (1.13): CaO(s) + H₂O(l) → Ca(OH)₂(aq) + Heat
Other Examples:
- C(s) + O₂(g) → CO₂(g) (burning of coal)
- 2H₂(g) + O₂(g) → 2H₂O(l)
- CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g) (natural gas)
Exothermic Reaction: Heat released (reaction mixture warms up).
Respiration is exothermic:
C₆H₁₂O₆(aq) + 6O₂(aq) → 6CO₂(aq) + 6H₂O(l) + Energy
Whitewashing: Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l) (shiny finish after 2–3 days)
Decomposition Reaction
Single substance → two or more simpler substances
General: AB → A + B (opposite of combination)
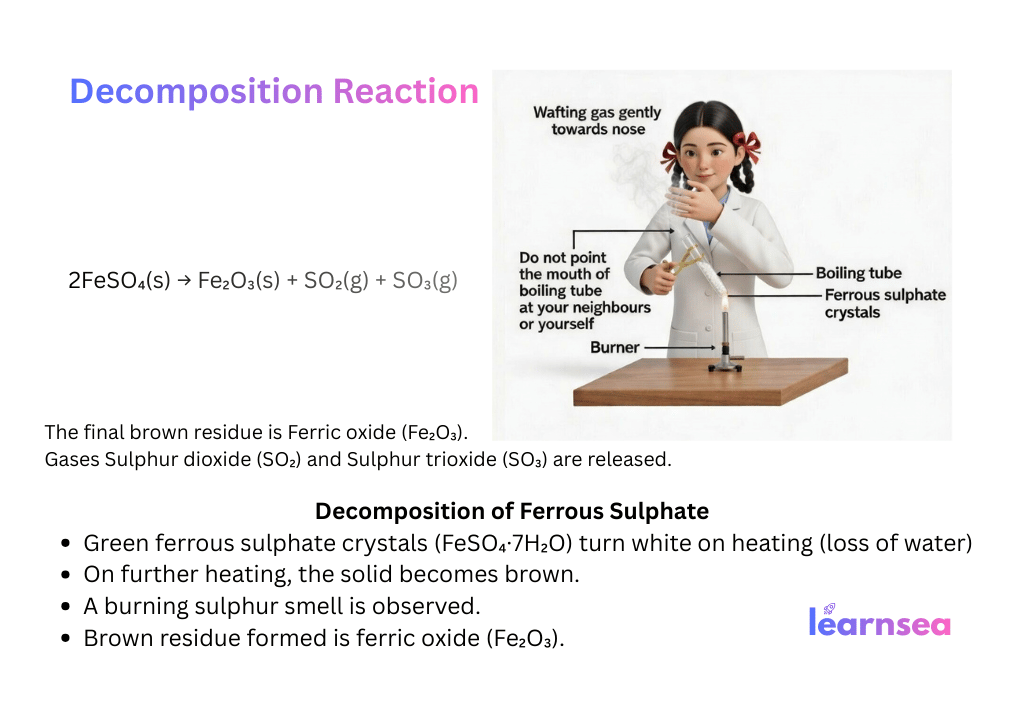
Activity 1.5 (Thermal):
2FeSO₄(s) →[Heat] Fe₂O₃(s) + SO₂(g) + SO₃(g)
(Green crystals → brown + smell of burning sulphur)
Activity 1.6 (Thermal):
2Pb(NO₃)₂(s) →[Heat] 2PbO(s) + 4NO₂(g) + O₂(g) (brown fumes)
Thermal Decomposition
CaCO₃(s) →[Heat] CaO(s) + CO₂(g) (used in cement)
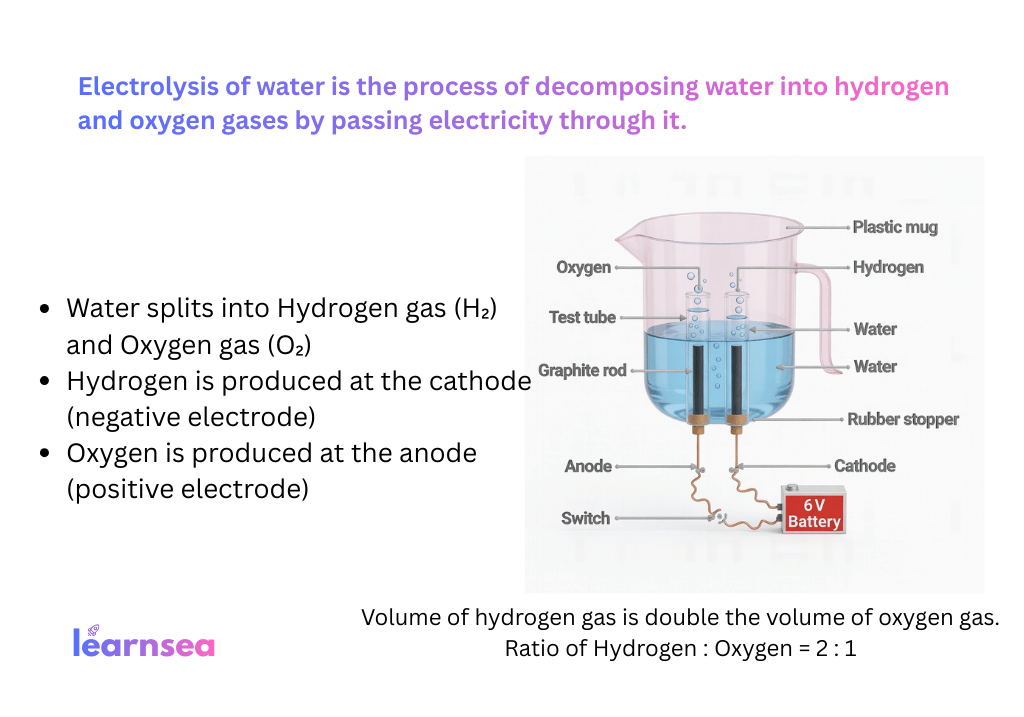
Activity 1.7 (Electrolytic – Electrolysis of water):
2H₂O(l) →[Electricity] 2H₂(g) + O₂(g)
(H₂ at cathode – double volume, O₂ at anode)
Activity 1.8 (Photolytic):
2AgCl(s) →[Sunlight] 2Ag(s) + Cl₂(g) (white → grey)
2AgBr(s) →[Sunlight] 2Ag(s) + Br₂(g) (used in black & white photography)
Endothermic Reaction: Energy absorbed (heat/light/electricity).
Displacement Reaction
More reactive element displaces less reactive element.
General: A + BC → AC + B
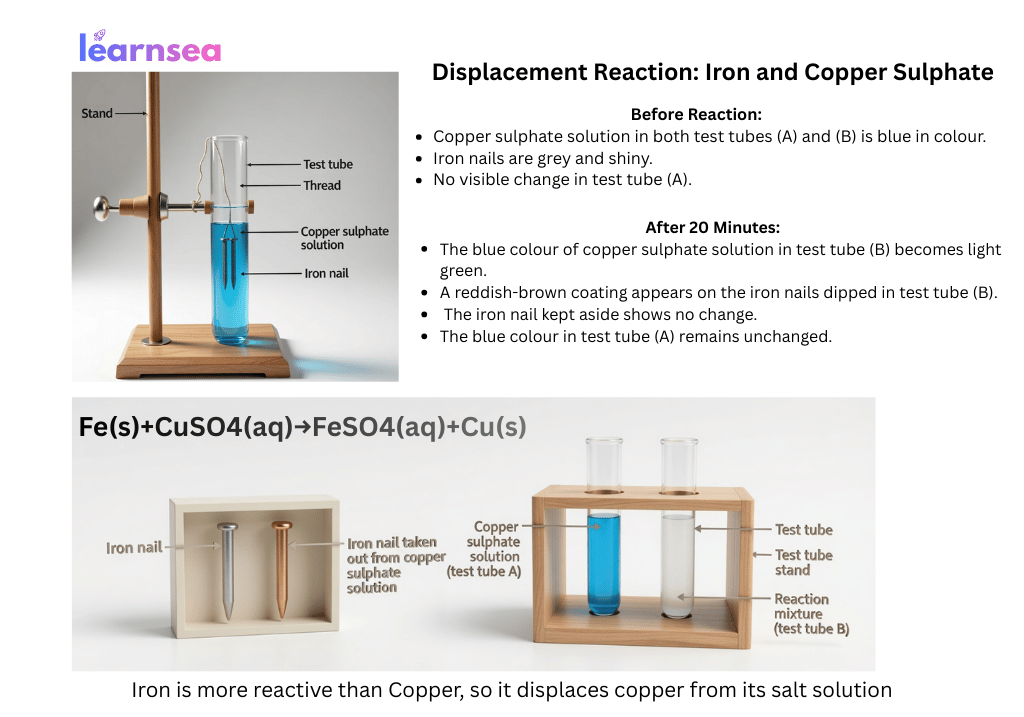
Activity 1.9:
Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
(Blue solution fades, iron nail becomes reddish-brown)
Reactivity Series based (Zn > Fe > Pb > Cu):
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
Pb(s) + CuCl₂(aq) → PbCl₂(aq) + Cu(s)
Double Displacement Reaction
Exchange of ions between two compounds.
General: AB + CD → AD + CB
Activity 1.10 (Precipitation Reaction):
Na₂SO₄(aq) + BaCl₂(aq) → BaSO₄(s)↓ (white ppt) + 2NaCl(aq)
Recall Activity 1.2: Pb(NO₃)₂ + 2KI → PbI₂(s)↓ (yellow ppt) + 2KNO₃ (also double displacement)
Oxidation and Reduction (Redox Reactions)
Activity 1.11:
2Cu(s) + O₂(g) →[Heat] 2CuO(s) (black coating)
CuO(s) + H₂(g) →[Heat] Cu(s) + H₂O(l) (black → reddish-brown)
Definitions:
- Oxidation: Gain of oxygen / Loss of hydrogen
- Reduction: Loss of oxygen / Gain of hydrogen
- Redox Reaction: Both occur simultaneously
Examples:
- ZnO + C → Zn + CO (ZnO reduced, C oxidised)
- MnO₂ + 4HCl → MnCl₂ + 2H₂O + Cl₂ (HCl oxidised, MnO₂ reduced)
Recall Activity 1.1: Mg is oxidised to MgO.
Effects of Oxidation Reactions in Everyday Life
Corrosion
Metal attacked by moisture, acids, oxygen → deterioration.
- Iron → Rust (Fe₂O₃·xH₂O) reddish-brown
- Silver → Black (Ag₂S)
- Copper → Green (basic copper carbonate)
Damage: Bridges, ships, cars, railings – huge economic loss.
Prevention: Paint, galvanising, oiling, alloying (stainless steel).
Rancidity
Oxidation of fats/oils in food → bad smell & taste.
Prevention:
- Antioxidants (BHA, BHT)
- Air-tight containers
- Flush chips packets with N₂ gas
- Keep in refrigerator
Frequently Asked Questions (FAQs)
What is a chemical reaction?
A chemical reaction is a process in which one or more substances (reactants) change into new substances (products) with different properties.
Why is it important to balance a chemical equation?
A chemical equation must be balanced to follow the law of conservation of mass, which states that mass can neither be created nor destroyed in a chemical reaction.
What are the main types of chemical reactions in Chapter 1?
The main types are:
- Combination Reaction
- Decomposition Reaction
- Displacement Reaction
- Double Displacement Reaction
- Oxidation and Reduction (Redox Reaction)
What is the difference between oxidation and reduction?
- Oxidation: Gain of oxygen or loss of hydrogen.
- Reduction: Loss of oxygen or gain of hydrogen.
Both occur together in a redox reaction.
What are corrosion and rancidity?
- Corrosion is the gradual destruction of metals by reaction with air and moisture (e.g., rusting of iron).
- Rancidity is the spoilage of food containing fats and oils due to oxidation, leading to bad smell and taste.
Conclusion
Chapter 1 Chemical Reactions and Equations form the backbone of Class 10 Chemistry. Understanding how chemical reactions occur, how to write and balance chemical equations, and how to identify different types of reactions builds a strong conceptual base for the entire syllabus.
The quick revision notes provided above are specially designed to help students revise important concepts in a short time. With clear explanations, key definitions, and important examples, these notes make last-minute preparation easy and effective.


