Class 10 Chemistry plays a crucial role in shaping a student’s understanding of core scientific concepts. From balancing chemical equations to identifying reaction types and observing colour changes, this syllabus lays the groundwork for advanced chemistry in higher classes. Class 10 Chemistry Important Reactions for quick revision
In this blog, you’ll find a complete collection of all the important reactions from the Class 10 Chemistry Important Reactions, presented in a clear and exam-focused format. Key equations, reaction types, common observations, and frequently asked concepts are compiled to help you revise efficiently and strengthen conceptual clarity.
Whether you’re preparing for board exams or doing a quick revision before a class test, this Chemistry Important Reactions article brings everything together in one place for systematic and confident preparation.
Chapter 1: Chemical Reactions and Equations
Definitions
- Chemical reaction: Process in which new substances with new properties are formed by rearrangement of atoms.
- Combination reaction: Two or more substances combine to form a single substance.
- Decomposition reaction: A single compound splits into two or more simpler substances (thermal, electrolytic, photolytic).
- Displacement reaction: One element displaces another from its compound.
- Double displacement reaction: Exchange of ions between two compounds (also called precipitation or neutralisation).
- Oxidation: Addition of oxygen or removal of hydrogen.
- Reduction: Removal of oxygen or addition of hydrogen.
- Redox reaction: Oxidation + reduction occur together.
- Exothermic: Heat released (+ heat on product side).
- Endothermic: Heat absorbed (+ heat on reactant side).
- Precipitate: Insoluble solid formed.
- Rancidity: Oxidation of fats/oils causing bad smell/taste.
- Corrosion: Oxidation of metals (e.g., rusting).
Key Reactions & Observations
| Reaction | Equation | Type | Observations/Color Change |
| Magnesium burns in air | 2Mg(s) + O₂(g) → 2MgO(s) | Combination | White powder forms |
| Zinc + dil. H₂SO₄ | Zn(s) + H₂SO₄(aq) → ZnSO₄(aq) + H₂(g) | Displacement | Heat evolved; H₂ gas (pop sound) |
| Lead nitrate heated | 2Pb(NO₃)₂(s) → 2PbO(s) + 4NO₂(g) + O₂(g) | Thermal decomposition | Yellow residue; brown fumes |
| Ferrous sulphate heated | 2FeSO₄(s) → Fe₂O₃(s) + SO₂(g) + SO₃(g) | Thermal decomposition | Green → brown; burning sulphur smell |
| Potassium iodide + lead nitrate | Pb(NO₃)₂(aq) + 2KI(aq) → PbI₂(s) + 2KNO₃(aq) | Double displacement | Yellow precipitate (colourless → yellow) |
| Ferrous sulphate + NaOH | FeSO₄(aq) + 2NaOH(aq) → Fe(OH)₂(s) + Na₂SO₄(aq) | Double displacement | Green precipitate |
| Barium chloride + H₂SO₄/Na₂SO₄ | BaCl₂(aq) + H₂SO₄(aq) → BaSO₄(s) + 2HCl(aq) | Double displacement | White precipitate |
| Copper + oxygen + water (rusting-like) | 2Cu(s) + O₂(g) + H₂O(l) → Cu(OH)₂(s) or basic carbonate | Oxidation | Reddish-brown Cu → green |
| Iron + copper sulphate | Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s) | Displacement | Blue → pale green; reddish-brown Cu deposit |
| Hydrogen + copper oxide | H₂(g) + CuO(s) → Cu(s) + H₂O(l) | Redox (reduction of CuO) | Black → reddish-brown |
| Quicklime + water | CaO(s) + H₂O(l) → Ca(OH)₂(aq) + heat | Combination (exothermic) | Vigorous heat evolution |
| Photosynthesis | 6CO₂(g) + 6H₂O(l) → C₆H₁₂O₆(aq) + 6O₂(g) | Endothermic (light) | — |
| Silver chloride in sunlight | 2AgCl(s) → 2Ag(s) + Cl₂(g) | Photodecomposition | White → grey |
Common indicators of reaction: Evolution of gas, formation of precipitate, change in colour, change in temperature, change in state.
Chapter 2: Acids, Bases and Salts
Definitions
- Acid: Substance that produces H⁺ ions in water, turns blue litmus red, sour taste.
- Base/Alkali: Produces OH⁻ ions, turns red litmus blue, bitter taste.
- Salt: Formed by acid + base (neutralisation).
- Neutralization: Acid + base → salt + water + heat.
- pH: <7 acidic, 7 neutral, >7 basic.
- Indicators: Dyes that show colour change in acid/base.
Indicator Colour Changes
| Indicator | Colour in Acid | Colour in Base/Alkali |
| Litmus | Red | Blue |
| Methyl orange | Red | Yellow |
| Phenolphthalein | Colourless | Pink |
| Red cabbage extract | Red/pink | Green |
| Turmeric | Yellow | Red-brown |
| Universal indicator | Red–orange (strong–weak acid) | Green–blue–purple (neutral–strong base) |
Key Reactions
- Acid + Metal (active metals): Zn/Mg/Fe/Al + dil. HCl/H₂SO₄ → salt + H₂(g) (pop sound)
Example: Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g) - Acid + Metal carbonate/hydrogencarbonate: Na₂CO₃/NaHCO₃ + HCl → salt + CO₂(g) + H₂O (brisk effervescence; CO₂ turns lime water milky)
NaHCO₃(s) + HCl(aq) → NaCl(aq) + CO₂(g) + H₂O(l) - Acid + Metal oxide (basic): CuO(s) + 2HCl(aq) → CuCl₂(aq) + H₂O(l) (black → blue-green solution)
- Neutralisation: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) + heat
- Chlor-alkali process (electrolysis of brine): 2NaCl(aq) + 2H₂O(l) → 2NaOH(aq) + Cl₂(g) + H₂(g)
- Baking soda + acid (in baking powder): NaHCO₃ + tartaric/citric acid → CO₂(g) (dough rises)
- Plaster of Paris setting: CaSO₄·½H₂O + 1½H₂O → CaSO₄·2H₂O (sets hard)
- Copper sulphate hydration: White anhydrous CuSO₄ + 5H₂O → blue CuSO₄·5H₂O (colour change white → blue on heating reverses)
Chapter 3: Metals and Non-metals
Definitions
- Metals: Lustrous, malleable, ductile, good conductors, electropositive.
- Non-metals: Non-lustrous, brittle, bad conductors, electronegative.
- Reactivity series: K > Na > Ca > Mg > Al > Zn > Fe > Sn > Pb > H > Cu > Hg > Ag > Au (more reactive displaces less reactive).
- Corrosion: Slow oxidation of metals by air/moisture (rusting of iron).
- Alloy: Homogeneous mixture of metals (e.g., brass, stainless steel) to prevent corrosion.
Key Reactions & Color Changes
- Metal + Oxygen (basic oxides):
4Na + O₂ → 2Na₂O (white)
2Mg + O₂ → 2MgO (white)
2Cu + O₂ → 2CuO (black coating) - Metal + Water/Steam:
2Na + 2H₂O → 2NaOH + H₂ (violent, H₂ catches fire)
3Fe + 4H₂O(g) → Fe₃O₄ + 4H₂ (steam) - Metal + Dil. Acid: Mg/Fe/Zn + dil. HCl/H₂SO₄ → salt + H₂(g) (pop sound)
- Displacement reactions (color changes):
Zn + CuSO₄(aq): Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
Observation: Blue solution → colourless; reddish-brown Cu deposit on Zn.
Fe + CuSO₄: Blue → pale green; reddish-brown Cu.
Cu + 2AgNO₃: Solution → blue; greyish-white Ag deposit on Cu. - Rusting of iron: 4Fe + 3O₂ + 2xH₂O → 2Fe₂O₃·xH₂O (rust)
Observation: Grey iron → red-brown flaky rust. - Aluminium corrosion: Forms protective Al₂O₃ layer (no further corrosion).
- Copper corrosion: Green basic copper carbonate.
- Silver tarnishing: 2Ag + H₂S → Ag₂S (black) + H₂.
Thermite reaction (exothermic): Fe₂O₃ + 2Al → 2Fe + Al₂O₃ + heat (molten iron).
Chapter 4: Carbon and its Compounds
Definitions
- Covalent compounds: Electron sharing (e.g., all carbon compounds).
- Hydrocarbons: Only C & H (saturated alkanes CnH2n+2; unsaturated alkenes CnH2n, alkynes CnH2n–2).
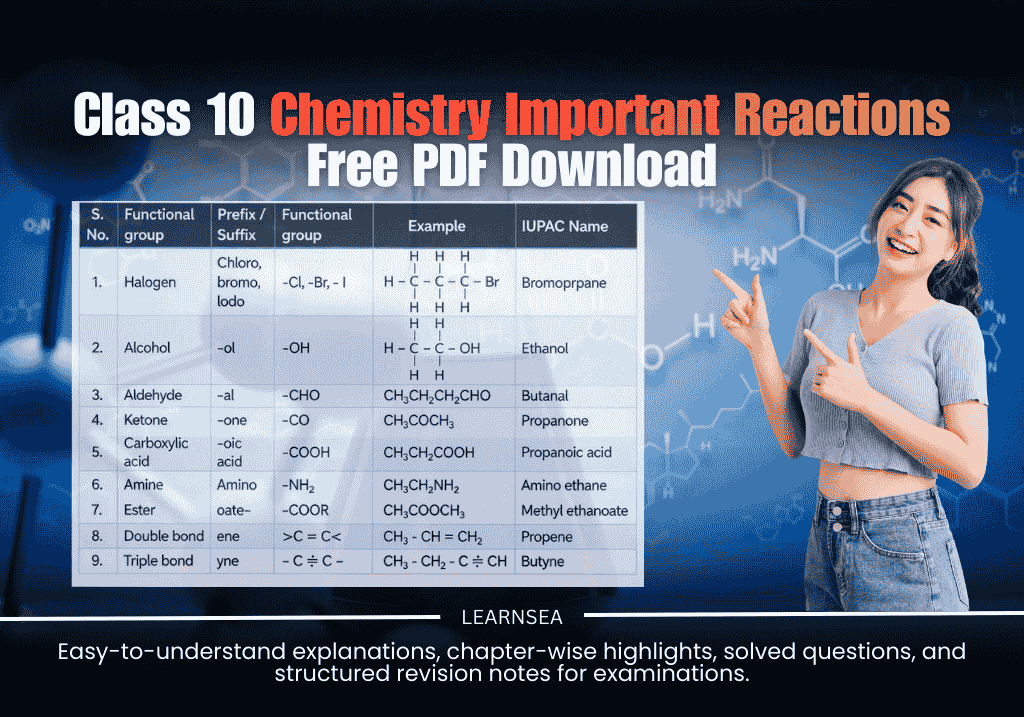
- Functional group: Determines properties (–OH alcohol, –COOH carboxylic acid, etc.).
- Combustion: Burning → CO₂ + H₂O + heat/light.
- Oxidation: Addition of O or removal of H (e.g., alcohol → acid).
- Addition reaction: Unsaturated compounds + H₂/Br₂/H₂O.
- Substitution reaction: Saturated compounds (alkanes + Cl₂ in sunlight).
- Esterification: Acid + alcohol → ester + water (sweet smell).
- Saponification: Ester + NaOH → soap + alcohol.
Key Reactions & Color Changes
- Combustion: CH₄ + 2O₂ → CO₂ + 2H₂O (clean flame); C₂H₂ + 5/2O₂ → 2CO₂ + H₂O (sooty flame).
- Oxidation of ethanol (with alkaline KMnO₄ or acidified K₂Cr₂O₇):
C₂H₅OH → CH₃COOH
Observation: Purple KMnO₄ decolourises; orange K₂Cr₂O₇ → green. - Dehydration of ethanol (conc. H₂SO₄, 170°C): C₂H₅OH → C₂H₄ + H₂O.
- Hydrogenation (addition): C₂H₄ + H₂ → C₂H₆ (Ni catalyst).
- Bromine water test (addition, color change):
Ethene/Ethyne + Br₂ water → decolourises (unsaturated).
Alkanes: No change (saturated). - Ethanol + Na: 2C₂H₅OH + 2Na → 2C₂H₅ONa + H₂(g) (pop sound).
- Ethanoic acid + NaHCO₃/Na₂CO₃: CH₃COOH + NaHCO₃ → CH₃COONa + H₂O + CO₂(g) (brisk effervescence; lime water milky).
- Esterification: CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ (ethyl acetate, sweet smell) + H₂O (conc. H₂SO₄).
- Soap making (saponification): Fat/oil + NaOH → soap (sodium stearate) + glycerol.
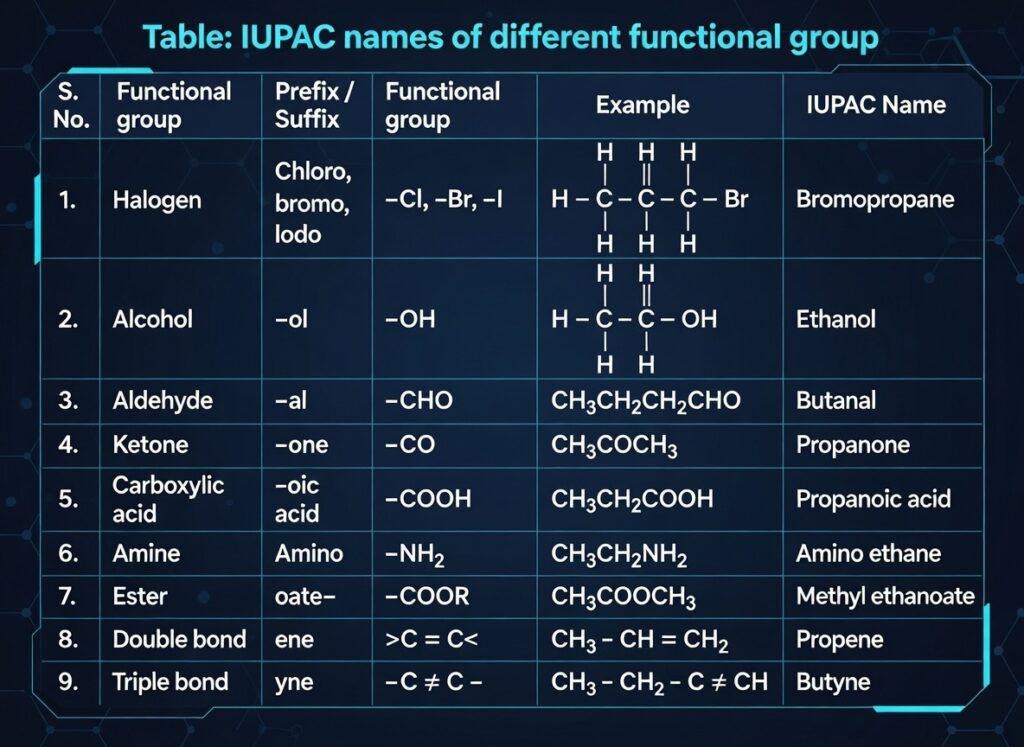
Litmus test: Carboxylic acids turn blue litmus red; alcohols do not.
Conclusion
In conclusion, understanding key reactions and functional groups is the key to scoring high in Class 10 Chemistry. Focus on concepts, revise regularly, and practice balanced equations to build confidence and accuracy for your board exams.