Are you searching for Chapter 1 Matter In our surrounding notes? In this article, we will provide the best NCERT class 9 notes. These notes can be used by students of class 9 to score high marks in class tests. Matter in our surrounding NCERT is an important chemistry chapter that enables students to think about the smallest particle called matter. The chapter deals with the properties of matter, Latent heat, Evaporation, Sublimation, Fusion, Condensation, and Freezing.
What is matter?
Matter is made of small particles that have mass and occupy space. Everything in the entire universe is made up of matter – the sun, planets, trees, animals, atmosphere, oceans, rivers, and so on. Many philosophers in the past argued that matters are the smallest particle that can’t be divided further. In 1968, Gabriele Veneziano gave string theory by using the Euler Beta Function. Matter is the smallest particle that consists of definite mass and has intermolecular space between them. Matters are very minute particles that a human can’t see with his naked eyes.
The characteristics of Particles of Matter | NCERT class 9 chapter 1 chemistry notes
- Particles of matter are continuously moving: The Particles of matter continually move due to kinetic energy. When temperatures rise then particles of matter move much faster. When temperatures decrease the particles of matter move at a slower rate.
- The particles of matter have space between them: The particles of matter have intermolecular space between them. For example, when we mix sugar (Salute) in a class of water then the sugar completely mixes in water (Solvent) without raising the level of water. This experiment clearly shows that particles of matter have space between them.
- Particles of matter are in contact with each other: the particles of matter attract with each other due to a strong force of attraction. Based on attraction the particle of matter is categorized into three physical states of matter – Solid, Liquid, and Gas.
- The intermolecular space between the particles of a solid is strongly attracted to each other due to minimum kinetic energy—intermediate in liquid and maximum in Gas.
- The force of attraction in solid is much stronger in the solid state, the intermediate force of attraction in liquid and less in gas.
- Due to low kinetic energy the movement of particles is minimum in solids, more in liquid, and maximum in gases
The states of matter
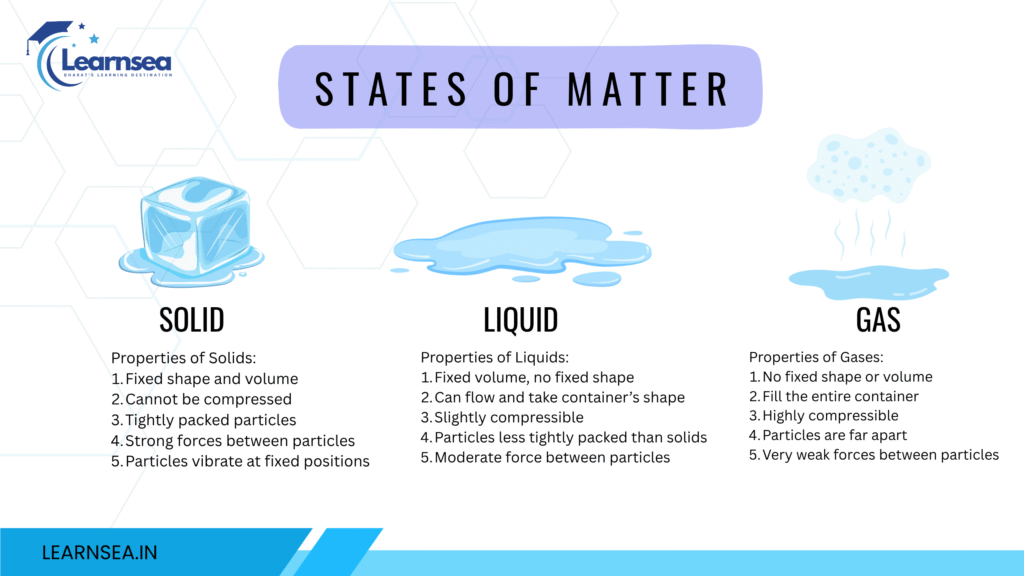
The physical state of matter is divided into three categories – Solid, Liquid, and gas.
- The teeth, bones, and muscles are solid in the human body.
- The blood in the human body, Acid in the Human digestive system, and water present in the human body are liquids.
- The exchange of gases takes place in the human lungs.
PROPERTIES OF SOLID
- The solid state of matter has a definite shape, and size, maintains rigidity, and does not flow, particles of matter are closely bounded with a strong force of attraction, and solids are generally incompressible. The example of solid state are Tables and chairs.
- Some exceptional cases: As we know rubber bands are solid but they can change it’s shape and size if we try to stretch them. When the external force stops the runner band regains its original shape and size. If external force exceeds then the rubber band may break.
- The sponge is solid. The sponge is filled with air to maintain its definite shape. We compress the sponge then it may get compressed and change its shape and size. If we remove the force, then the sponge gets its original shape and size.
- The sugar, salts, pulses, rice, and wheat take the shape of the container in which they are placed. They have definite shapes and sizes which is why they are solid.
PROPERTIES OF LIQUID
- The force of attraction between the particles keeps the particles together and keeps the volume the same.
- Liquids take the shape of the container in which they are kept.
- Liquid has a fixed volume but not a fixed shape due to the property of fluid.
- In nature, the oxygen and carbon dioxide can easily diffuse in liquid. This property enables the air to dissolve into liquids. This process supports the aquatic animals and plants to survive. Terrestrial plants and animals can exchange gases in plant parts and transportation of oxygen through Hemoglobin in blood streams respectively.
- The rate of diffusion is much higher in liquid than in solid due to kinetic energy and intermediate intermolecular space between them.
PROPERTIES OF GAS
- The gas state of matter has no fixed shape, nor fixed volume, the particles between gasses are loosely packed due to the weak force of attraction. The gases are highly compressible for example helium gases in balloons and LPG gas for domestic use.
- The gases can flow due to low force of attraction and maximum kinetic energy between the particles of matter.
- Gases are freely moveable to free space available to them and can flow (have fluidity).
- The pressure of gasses is the force applied on the walls of the container in which they are kept due to irregularly moving gas particles.
- The change of the physical state of matter can be done in two ways
Class 9 Chemistry Chapter 1 Structure of Atom Notes
Melting point
When a solid substance melts at certain temperatures then it is called melting point. The melting point of various substances depends on temperature. The melting of ice is 0°C Or 273.16 K. Whenever the solid melts the temperature of the substance doesn’t rise due to the latent heat of fusion.
Whenever the solid melts the temperature of the substance doesn’t rise due to the latent heat of fusion. Above 100 degrees of water vapor greater energy than 100 degrees of water vapor has greater energy than 100 degrees Celsius of water. However, we can say that when we change the state of matter the temperature also changes. Generally, at zero degrees Celsius, the water freezes to solid ice, at 27 degrees Celsius the water remains in a liquid state, and above 100 degrees Celsius, the water transforms into a gaseous state 9 (steam).
Sublimation
When any solid substance like naphthalene (white solid) ball and ammonium chloride (White semi-solid) changes into a gaseous state called sublimation. When the gaseous state transforms back to solid in the form of crystals called deposition for example the water vapor in the air changes into ice in clouds and forms frost during low temperature.
How we can liquify atmospheric gases?
We can liquify atmospheric gases by increasing the pressure in a cylinder in which the distance between the particles gets reduced and comes together and by lowering the temperature for example the oxygen gases can be liquified by this process. The LPG and CNG cylinders are also filled with liquified and compressed. The dry Ice (carbon dioxide) changes directly into gas unaccompanied into liquid when pressure is reduced to Atmospheric pressure (101,325 Pa).
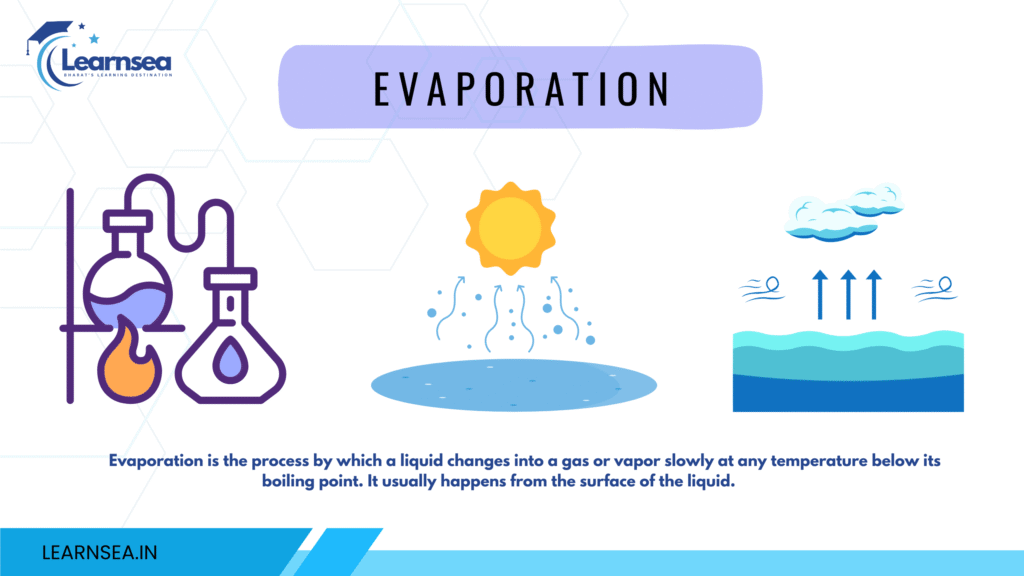
Evaporation
The natural phenomenon is where liquid changes into a gaseous state at any temperature. The matter (particle) on the surface of the fluid (liquid) has greater kinetic energy. Due to greater kinetic energy, the intermolecular force of attraction gets weakened and escapes into gases. Evaporation always causes a cooling effect in the surroundings. When we put acetone on our palms then it takes heat from our palms and vaporizes in the air. When we wear cotton clothes in summer it absorbs sweat and makes our bodies cool in summer. In many parts of India, people sprinkle water on terraces or sitting areas because the water absorbs enough heat from the ground and evaporates into the atmosphere. The factors that affect evaporation are as follows
- The Sudden Increase in temperature
- Exposed or large surface area
- Humidity or moisture in the air
- The speed of the wind